The Water Cycle
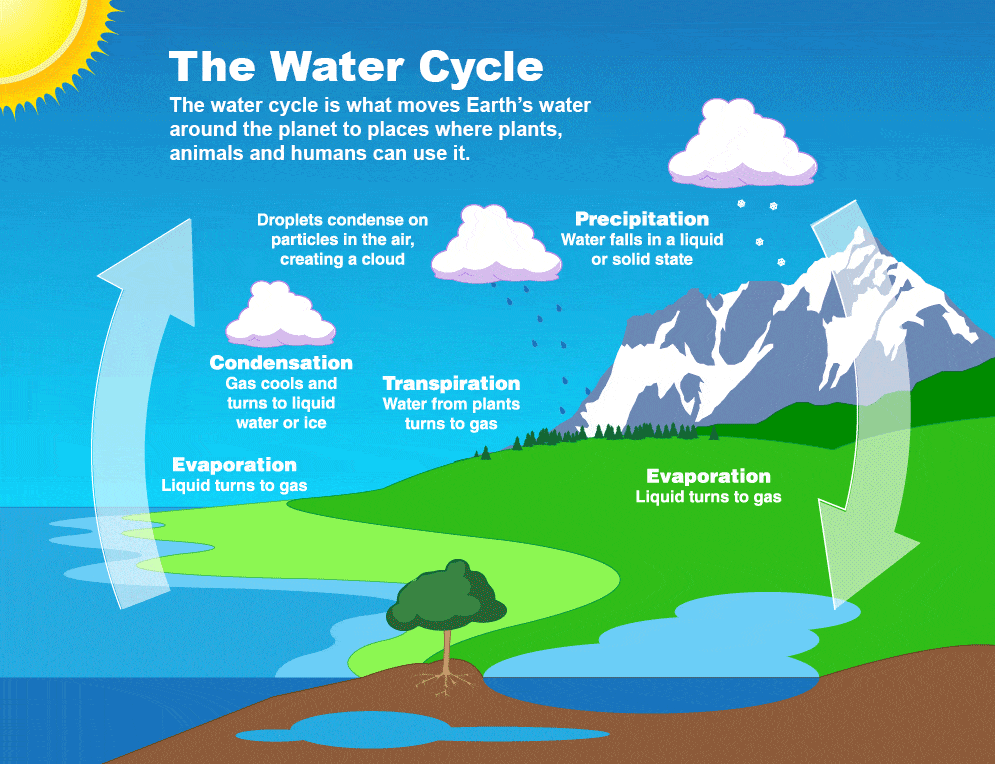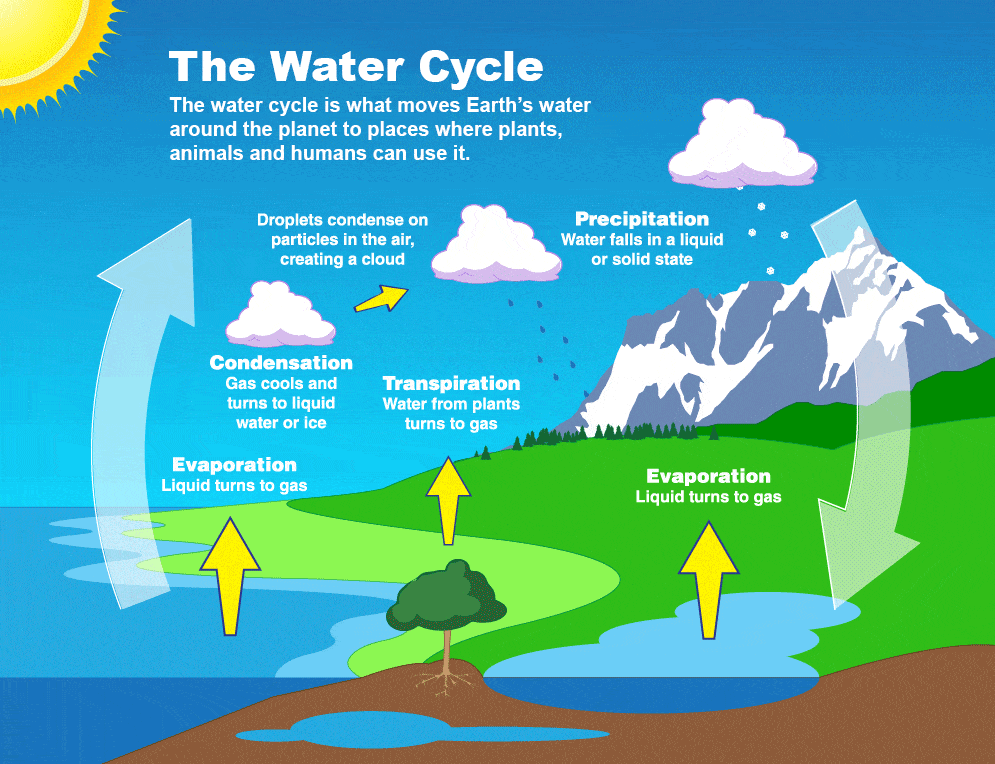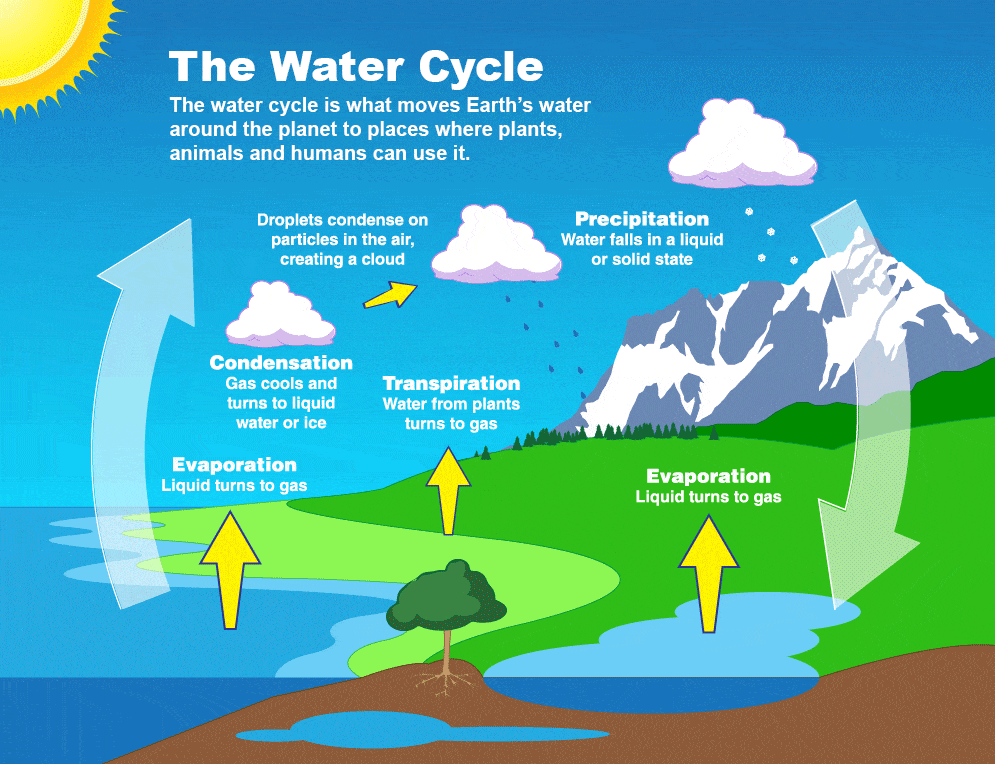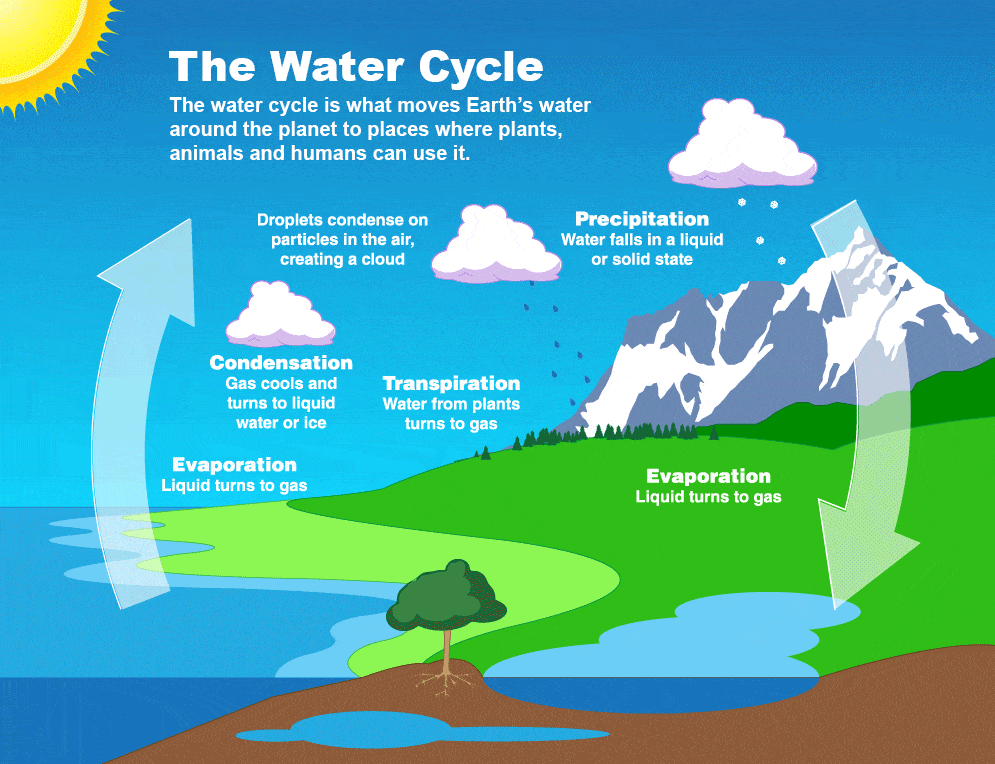
There is a consistent volume of water on Earth and in our atmosphere, but it is cycling continuously between a liquid, gas, and solid state. The water cycle helps us visualize how water moves up and down in the atmosphere and through our environment.
Precipitation refers to water falling from clouds down to the Earth's surface as a liquid (rain) or in its solid form (snow or hail). Once on the ground, water either soaks into the ground, or runs into waterways like rivers, lakes, and the ocean. Water soaked into the ground is taken up by the roots of plants or becomes groundwater. Through the process of photosynthesis, plants exhale or transpire water vapor which sends water back to the atmosphere.
Meanwhile, the sun heats water on the Earth's surface, causing it to evaporate into the atmosphere. Eventually, water vapor that has accumulated in the atmosphere cools, causing condensation that becomes precipitation again. And the cycle continues!
States of Water
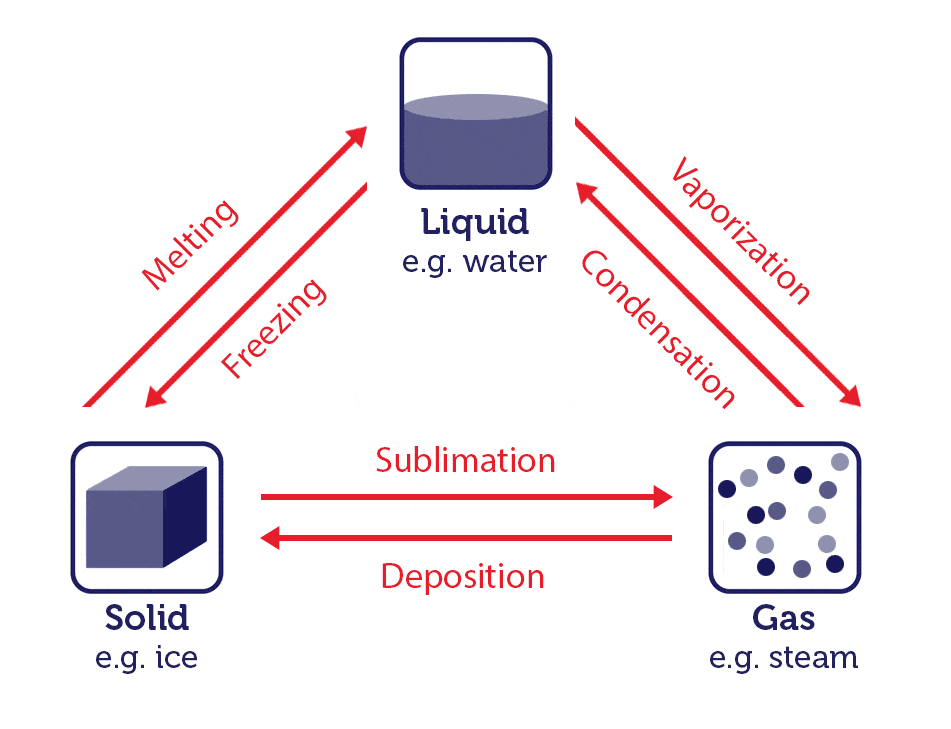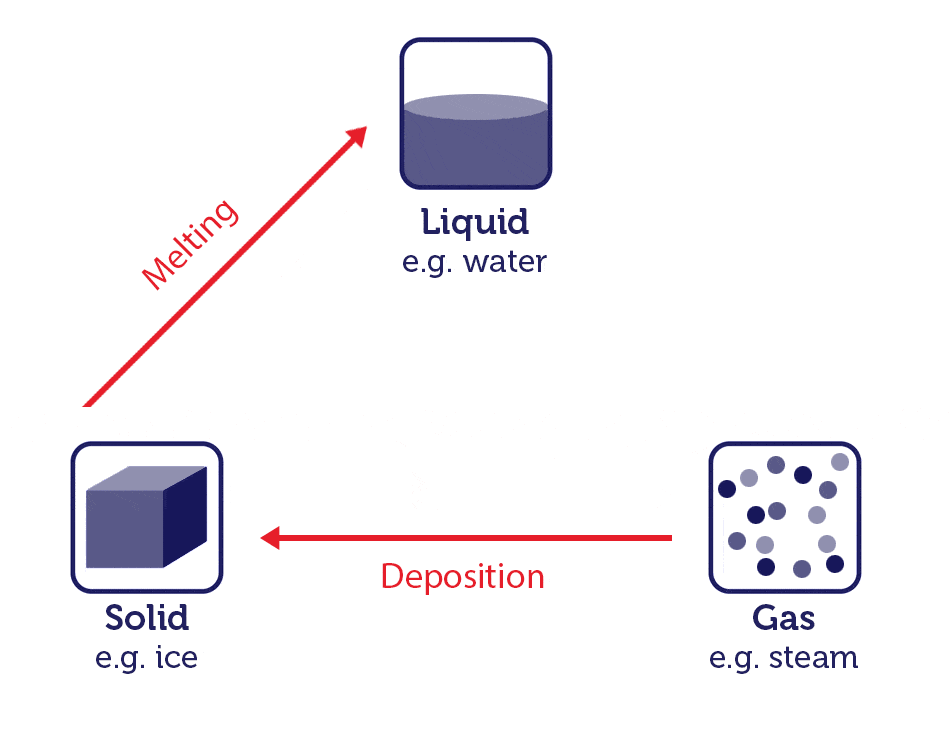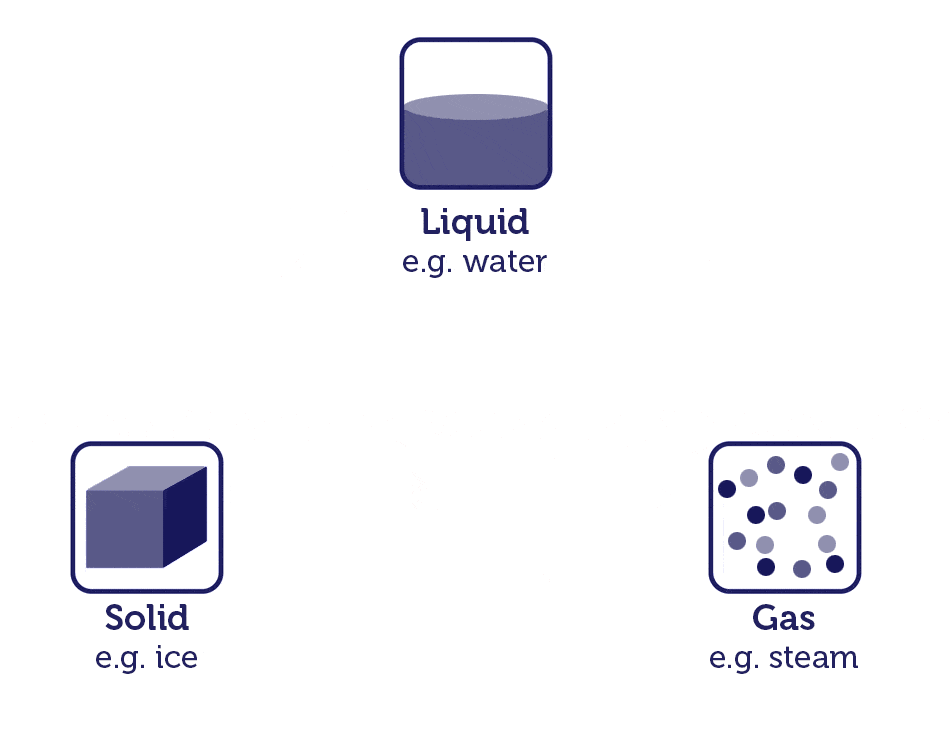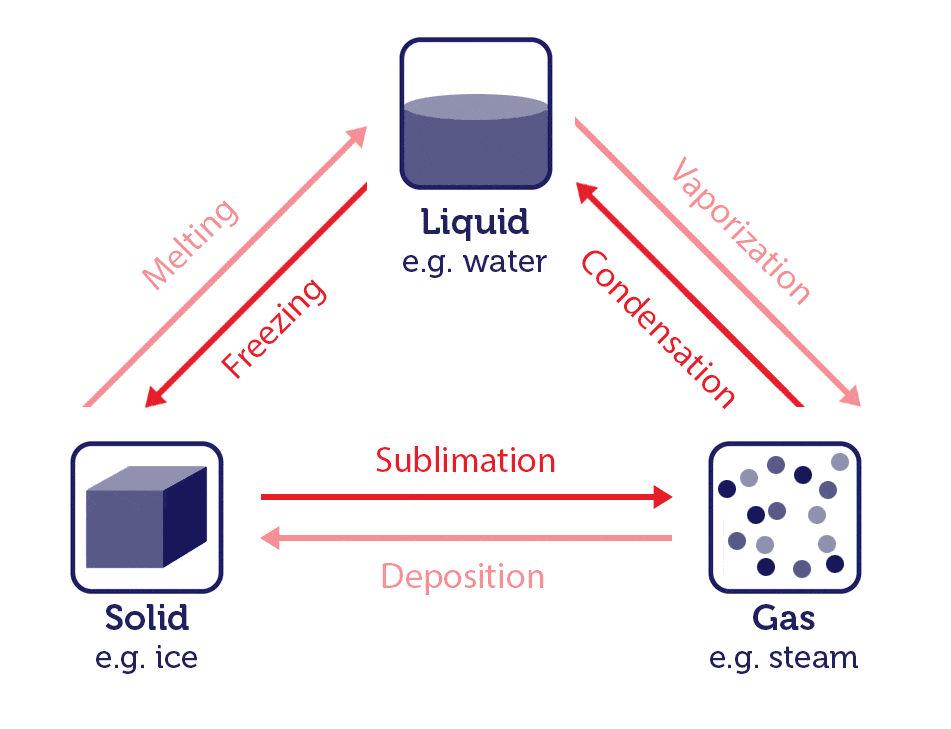
Water is the only substance on Earth that can be found in all three physical states under normal temperatures. We typically think of water in its liquid state, where it can move around and take the shape of the container it's in. When liquid water is heated up to the point where it boils - 212°F (100°C) - it vaporizes into its gas form. When that gas cools, it goes through the process of condensation and returns to its liquid form. When liquid water gets cold - 32°F (0°C) - it freezes into its solid form - ice or snow. When that solid warms back up, it melts back into its liquid form.
In less obvious situations, water can transform from its solid form to gas and back again. Think about frost on your window on a cold morning. Frost is an example of where water vapor in its gas form has frozen and stuck to the glass -- a process called deposition. The opposite is possible too. Imagine a really cold ice cream cone that you're holding on a warm day -- have you noticed a steam rising from the sugary goodness? This is an example of water as a solid transforming directly into a gas -- a process called sublimation.